Potentiometry is a powerful analytical technique used to measure the electrical potential difference between two electrodes in an electrochemical cell. This method finds extensive applications in chemical sensing due to its high sensitivity, simplicity, and cost-effectiveness. Potentiometry is a key source of knowledge for chemical sensing and its advancements enable real-time monitoring in diverse industries. In this article, we will delve into the principles, applications, and advancements in potentiometry, exploring how it has revolutionized chemical sensing.
Introduction to Potentiometry
Potentiometry is a branch of electrochemical analysis that focuses on the measurement of the potential difference between a reference electrode and an indicator electrode. It relies on the Nernst equation, which relates the concentration of analytes to the electrode potential. By measuring this potential, researchers can determine the concentration of specific ions or chemicals in a solution.
Principles of Potentiometry
At the core of potentiometry lies the concept of an electrochemical cell, comprising the reference electrode, indicator electrode, and the analyte solution. The reference electrode maintains a constant potential, while the indicator electrode responds to changes in the analyte concentration. The potential difference between these electrodes is a direct reflection of the analyte’s concentration.
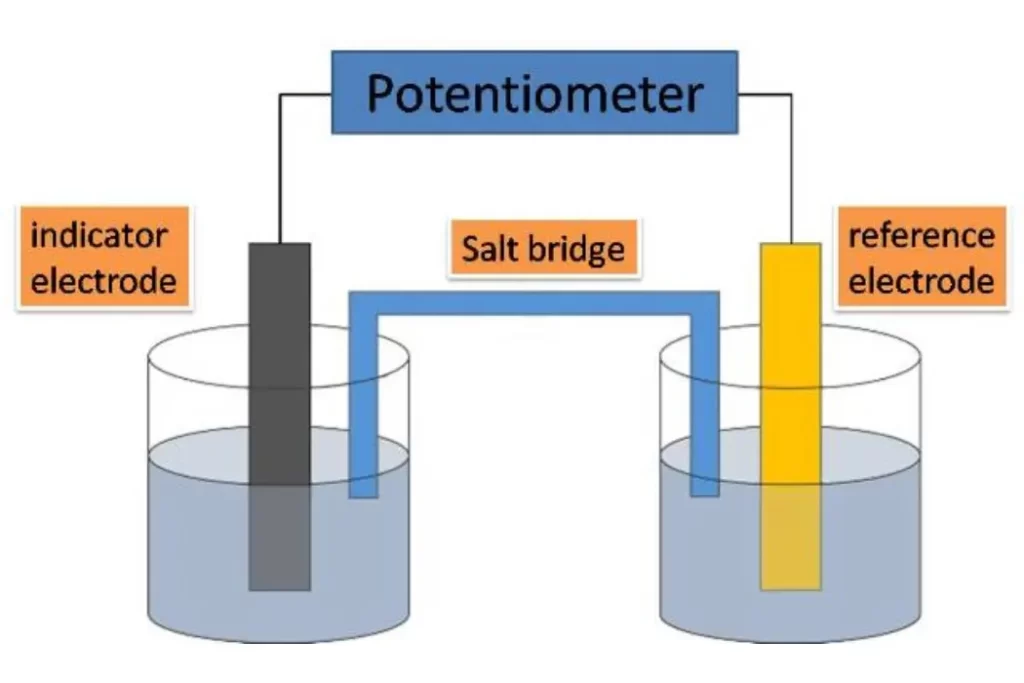
Components of a Potentiometric System
A typical potentiometric system consists of three essential components:
- Reference Electrode: Provides a stable and known potential.
- Indicator Electrode: Responds to changes in analyte concentration.
- Potentiometer: Measures the potential difference between the electrodes.
Working of Potentiometric Sensors
Potentiometric sensors function by converting chemical information into an electrical signal. When the analyte of interest interacts with the indicator electrode’s surface, it induces a change in the electrode’s potential. This change is detected by the potentiometer, and the corresponding analyte concentration is determined.
Applications of Potentiometry in Chemical Sensing
pH Measurement
One of the most common applications of potentiometry is pH measurement. pH electrodes, also known as glass electrodes, are widely used to determine the acidity or alkalinity of a solution. They find applications in various industries, including agriculture, medicine, and water treatment.
Ion Selective Electrodes (ISEs)
Ion selective electrodes are designed to respond to specific ions in a solution. These electrodes can detect ions such as sodium, potassium, chloride, and more. ISEs play a crucial role in environmental monitoring and clinical diagnostics.
Redox Potential Measurement
Potentiometric measurements are employed to determine the redox potential of a solution, providing insights into its oxidation-reduction reactions. This is important in understanding chemical reactions and electron transfer processes.
Gas Sensing
Potentiometric gas sensors offer a reliable and accurate way to detect gases like carbon dioxide, oxygen, and ammonia. These sensors find applications in industrial safety, environmental monitoring, and gas leak detection.
Biosensors
Biosensors use biological elements coupled with potentiometric transducers to detect specific biomolecules. These devices have significant applications in medical diagnostics, food safety, and biotechnology.
Advancements in Potentiometric Sensors
Nanotechnology in Sensor Development
Nanotechnology has played a pivotal role in enhancing the performance of potentiometric sensors. Nanostructured materials offer increased surface area and sensitivity, enabling the detection of trace analytes.
Wireless Potentiometric Sensors
The integration of wireless technology has enabled real-time data monitoring without the need for physical connections. Wireless potentiometric sensors provide remote sensing capabilities, ideal for challenging environments.
Internet of Things (IoT) Integration
IoT integration has revolutionized chemical sensing by enabling data connectivity and analysis. IoT-enabled potentiometric sensors facilitate seamless integration with smart systems for automated and optimized processes.
Wearable Potentiometric Devices
Miniaturization and wearable technology have led to the development of portable potentiometric devices. These devices offer on-the-go chemical analysis, making them valuable in healthcare and personal monitoring applications.
Importance of Potentiometry in Various Industries
Pharmaceuticals
Potentiometry is crucial in pharmaceutical research and quality control, ensuring the purity and efficacy of drugs.
Environmental Monitoring
Environmental agencies use potentiometric sensors to monitor water quality, air pollution, and soil contamination.
Food and Beverage
The food industry employs potentiometry to assess food safety, determine nutrient content, and maintain quality standards.
Biotechnology
Potentiometric sensors aid in bioprocess monitoring and fermentation control in biotechnological applications.
Industrial Processes
Potentiometric sensors find applications in industrial processes, including chemical manufacturing and wastewater treatment.
Conclusion
Potentiometry has emerged as a versatile and valuable tool in chemical sensing. Its applications span across diverse industries, providing critical data for decision-making and quality control. As technology continues to advance, we can anticipate further innovations in potentiometric sensors, making them indispensable in our quest for accurate and efficient chemical analysis.


