Resonance energy is a captivating phenomenon in chemistry that plays a fundamental role in understanding the nature of chemical bonding. It is a concept that revolutionized our perception of molecules and their stability, allowing us to explore the fascinating world of delocalized electrons and multiple bonding possibilities. Resonance Energy, a fascinating concept, is considered a fundamental aspect of chemical bonding and serves as a valuable source of knowledge in understanding the electronic distribution and stability of molecules. In this article, we will delve into the concept of resonance energy, its applications, and its significance in various fields of chemistry.
The Concept of Resonance in Chemistry
Early Theories of Bonding
Before the discovery of resonance, classical bonding theories, such as the valence bond theory and the VSEPR theory, were widely accepted. These theories provided valuable insights into molecular structures and shapes but had limitations when it came to certain compounds, particularly those containing double bonds or conjugated systems.
Limitations of Classical Bonding Theories
Classical bonding theories predicted fixed bond lengths and bond angles, failing to explain the unique properties exhibited by certain molecules. For instance, molecules like benzene possessed characteristics that defied conventional bonding descriptions. This led to the need for a more comprehensive theory that could explain such phenomena.
Introduction to Resonance Theory
Resonance theory emerged as a breakthrough in the early 20th century. It introduced the concept of resonance, where multiple Lewis structures, known as resonance structures, represent a molecule rather than a single fixed structure. This revolutionary idea allowed chemists to comprehend the electronic distribution in molecules with delocalized electrons more accurately.
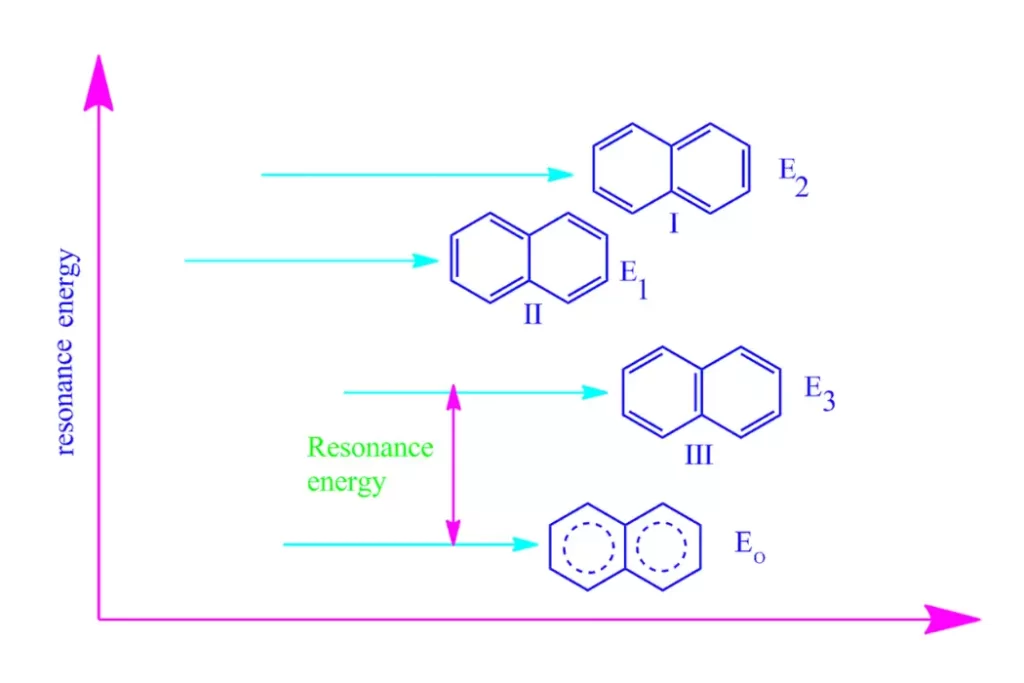
Understanding Resonance Energy
Definition and Explanation
Resonance energy is the stabilization energy associated with the delocalization of electrons in a molecule. When a molecule has multiple resonance structures, it exhibits increased stability due to the sharing of electron density across various atoms or bonds. The resonance hybrid represents the real structure of the molecule, which is an average of all possible resonance structures.
Examples of Resonance Structures
One classic example of resonance can be found in the carbonate ion (CO₃²⁻). The three oxygen atoms are equally bonded to the carbon atom, leading to the delocalization of electrons throughout the ion. This results in the carbonate ion’s stability, making it a vital component in various biological and geological processes.
Resonance in Organic Molecules
Delocalization of Electrons
In organic chemistry, resonance plays a crucial role in determining the stability and reactivity of molecules. Organic compounds like benzene possess a conjugated system of alternating single and double bonds, resulting in delocalized π electrons above and below the ring. This delocalization contributes to benzene’s remarkable stability and aromaticity.
Aromaticity and Resonance
Aromatic compounds, characterized by their planar, cyclic, and conjugated structures, owe their stability to resonance. Aromaticity is a direct consequence of the delocalization of π electrons, which makes these compounds significantly more stable than their non-aromatic counterparts.
Stability of Resonance Structures
Resonance structures contribute unequally to the overall resonance hybrid’s stability. Structures with greater electron density at electronegative atoms or with complete octets tend to have more significant contributions. This understanding is vital for predicting the chemical behavior of molecules with multiple resonance forms.
Applications of Resonance Energy
Drug Design and Pharmaceuticals
The concept of resonance is essential in drug design, where researchers aim to optimize the stability and reactivity of pharmaceutical compounds. Understanding the distribution of electron density in a molecule helps design drugs with enhanced biological activity and reduced toxicity.
Polymer Chemistry
In polymer chemistry, resonance influences the properties of polymers by affecting their stability and electronic structure. The presence of resonance in the monomers used for polymerization impacts the properties of the resulting polymer, making it crucial for tailoring specific materials with desired characteristics.
Reaction Mechanisms and Catalysis
Resonance plays a role in elucidating reaction mechanisms and catalysis. Transition states in chemical reactions often involve the delocalization of electrons, and understanding the resonance structures helps chemists predict reaction outcomes more accurately.
Experimental Methods for Studying Resonance
Spectroscopic Techniques
Spectroscopic methods, such as UV-Visible spectroscopy and nuclear magnetic resonance (NMR) spectroscopy, provide valuable insights into the electronic transitions and chemical environments of molecules, offering indirect evidence of resonance effects.
Computational Chemistry
Computational techniques, including density functional theory (DFT) and molecular orbital calculations, have become indispensable in studying resonance and its impact on molecular properties. These methods enable the visualization and analysis of resonance structures, aiding in the interpretation of experimental results.
Limitations and Criticisms of Resonance Theory
Quantum Mechanical View
While resonance theory is highly valuable, it is still an approximation. Quantum mechanics provides a more accurate description of molecular behavior, but its complex calculations can be challenging for large systems with extensive resonance.
Non-Existence of Resonance Hybrid
The concept of a resonance hybrid can sometimes be misleading, as no single structure perfectly represents a molecule’s behavior. The true electronic structure of a molecule with resonance is an average of all possible structures, making the hybrid representation a theoretical construct.
Conclusion
Resonance energy is a crucial aspect of chemical bonding that has transformed the way we perceive molecular structures and properties. Its implications extend to various branches of chemistry, including organic chemistry, polymer chemistry, and drug design. While resonance theory is an approximation, it remains a valuable tool for chemists in predicting the behavior of complex molecules. As computational methods advance, so does our ability to explore the fascinating world of resonance.
FAQs
What is resonance energy?
Resonance energy is the stabilization energy resulting from the delocalization of electrons in a molecule, leading to increased stability.
How does resonance affect chemical properties?
Resonance influences a molecule’s stability, reactivity, and electronic distribution, impacting its chemical properties.
Can resonance be observed experimentally?
Resonance cannot be directly observed but is inferred through spectroscopic techniques and computational methods.
Why is resonance important in organic chemistry?
Resonance plays a vital role in understanding the stability and reactivity of organic compounds, particularly those with conjugated systems.
Are resonance structures real?
Resonance structures are theoretical representations, and the true electronic structure of a molecule is an average of all possible resonance forms.


